Combination vaccines made by messenger RNA (mRNA) are the new way to go, according to Moderna president Dr. Stephen Hoge at the 2024 Goldman Sachs Global Healthcare Conference.
Dr. Hoge spoke briefly at the virtual webinar June 10 to explain the current information Moderna has on mRNA vaccines and their ideas for mRNA.
According to Dr. Hoge, these mRNA vaccines are versatile for treatment. They serve four purposes: treating respiratory illnesses, latent viruses, cancer, and activating or deactivating protein expression.
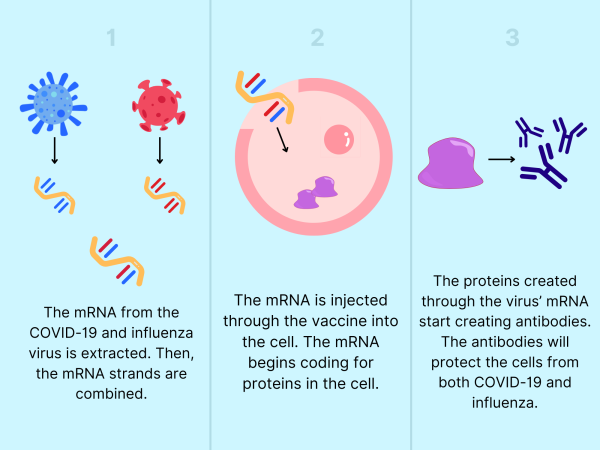
According to Dr. Hoge, Moderna is in the process of developing a combined COVID-19 and influenza vaccine, created with mRNA. Dr. Hoge said that a combination vaccine would be both cost- and health-efficient. The data from a Phase 3 clinical trial recently came back positive.
“We were able to demonstrate significantly higher titers against influenza [and] be what we believe is the best COVID component [in] that same clinical trial,” says Dr. Hoge. “It’s really a dream come true.”
While traditional vaccines are created by growing large amounts of infectious disease to deactivate, mRNA vaccines expedite the vaccine process.
“This vaccine gives your cells instructions on how to make components of the pathogen, which will incite an immunological response,” said Dr. Heta Javeri, an infectious disease specialist and program director of the Infectious Disease Fellowship at UT Health San Antonio. “Traditional vaccines use a live attenuated virus … in comparison, mRNA vaccines are ‘cleaner.’ ”
Vaccines created with mRNA are confirmed to be effective, but concerns about a combined COVID-19 and influenza vaccine loom. Both COVID-19 and influenza are susceptible to mutations and are constantly changing. Dr. Javeri acknowledges this as a vital concern.
“I think this is going to be one of the challenges of this specific combination vaccine. Every flu vaccine is different because of the antigen shifts and drifts that occur,” says Dr. Javeri. “Now, COVID does the same thing, but do they do it on the same timeline?”
Moderna is not the only pharmaceutical company developing a combination vaccine. “Pfizer [and] NovaVax are also moving toward this combination vaccine. I think they’re already in Phase 1 or 2,” Dr. Javeri says.
Because the combination vaccine is essentially a two-in-one, it is far cheaper to invest in rather than utilizing large funds for both the COVID-19 and influenza vaccine. According to Dr. Hoge, this allows Moderna to fund other projects.
“With $750 million, [we can] commit to fund the development which would provide the upcoming for us to invest in a different program,” Dr. Hoge says.
– July 22, 2024 –



Aayush • Jul 22, 2024 at 10:19 pm
Amazing writing! I loved the visual representation of how the vaccine works. Great work!
Dhara Agrawal • Jul 22, 2024 at 6:49 pm
This is so informative! Thanks for sharing